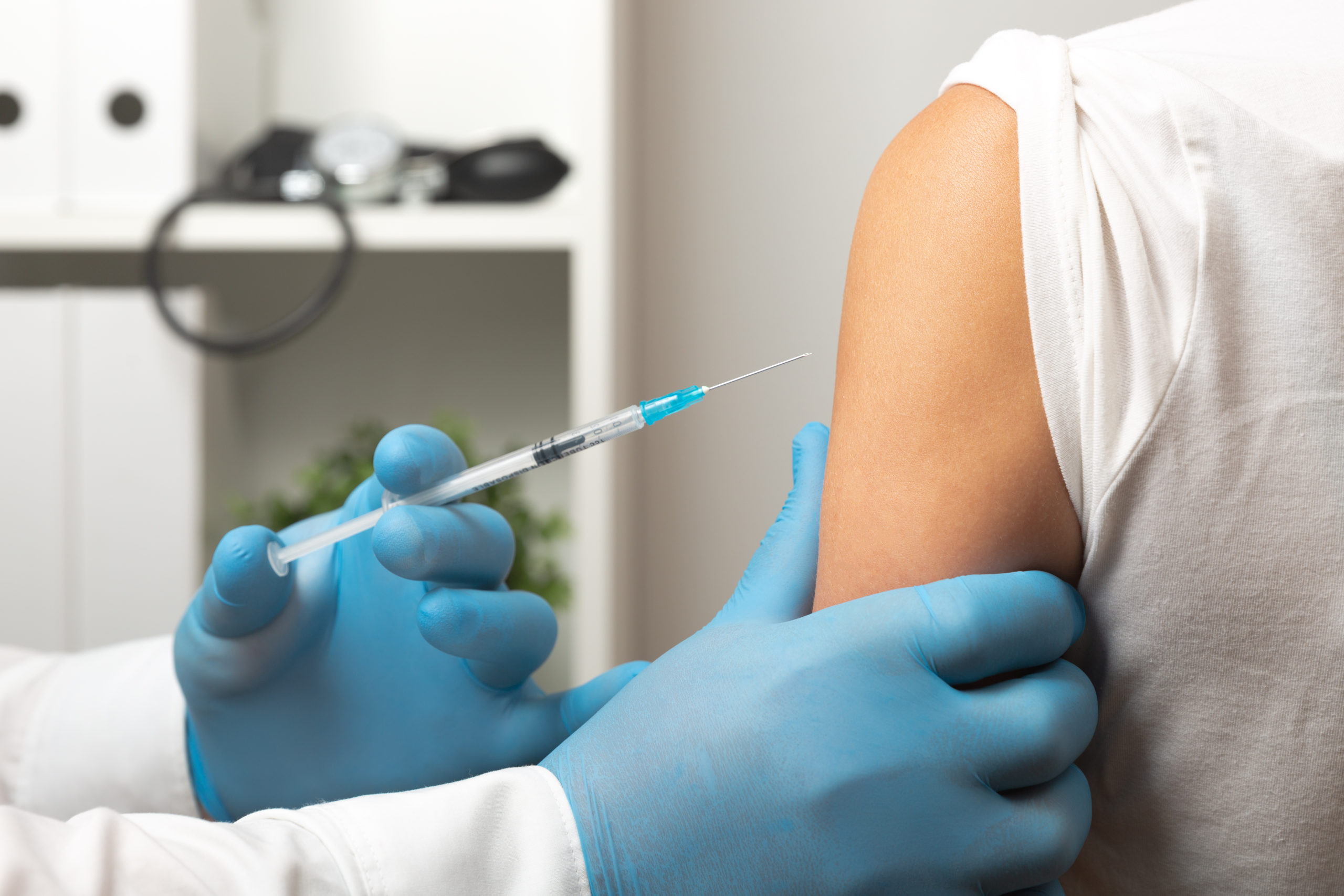
To be compensated in the Vaccine Program, an individual must establish a causal link between the vaccination and an injury…
Insights
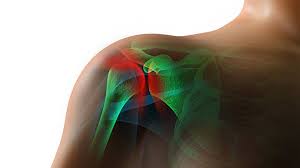
Shoulder Injury Related to Vaccine Administration (“SIRVA”) is a recognized vaccine injury. SIRVA symptoms typically include pain at or around…
Shoulder Injury Related to Vaccine Administration (“SIRVA”) is a recognized vaccine injury. SIRVA symptoms typically include pain at or around…
Shoulder Injury Related to Vaccine Administration (“SIRVA”) is a recognized vaccine injury. SIRVA symptoms typically include pain at or around…
The Vaccine Injury Table identifies vaccinations, injuries and time periods for the expected onset of the injury for eligible compensation…
Shoulder Injury Related to Vaccine Administration (“SIRVA”) is a recognized vaccine injury. SIRVA symptoms typically include pain at or around…
Shoulder Injury Related to Vaccine Administration, or “SIRVA,” is an increasingly common and well-recognized vaccine injury. Understanding more about this…
The Vaccine Program requires vaccine injured petitioners to satisfy a severity requirement. Generally, the severity requirement specifies how long an…
Filing a claim for compensation for a vaccine injury involves initiating a legal claim in the United States Court of…
Filing a claim in the National Vaccine Injury Compensation Program (“NVICP”) differs from filing a traditional lawsuit. Claims filed in…